- Record: found
- Abstract: found
- Article: found
Germline and somatic polymerase ε and δ mutations define a new class of hypermutated colorectal and endometrial cancers
Read this article at
Abstract
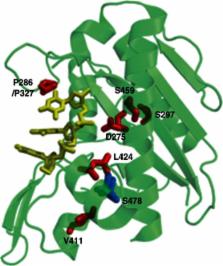
Polymerases ϵ and δ are the main enzymes that replicate eukaryotic DNA. Accurate replication occurs through Watson–Crick base pairing and also through the action of the polymerases' exonuclease (proofreading) domains. We have recently shown that germline exonuclease domain mutations (EDMs) of POLE and POLD1 confer a high risk of multiple colorectal adenomas and carcinoma (CRC). POLD1 mutations also predispose to endometrial cancer (EC). These mutations are associated with high penetrance and dominant inheritance, although the phenotype can be variable. We have named the condition polymerase proofreading-associated polyposis (PPAP). Somatic POLE EDMs have also been found in sporadic CRCs and ECs, although very few somatic POLD1 EDMs have been detected. Both the germline and the somatic DNA polymerase EDMs cause an ‘ultramutated’, apparently microsatellite-stable, type of cancer, sometimes leading to over a million base substitutions per tumour. Here, we present the evidence for POLE and POLD1 as important contributors to the pathogenesis of CRC and EC, and highlight some of the key questions in this emerging field. Copyright © 2013 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd
Related collections
Most cited references15
- Record: found
- Abstract: found
- Article: not found
Germline mutations in the proof-reading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas
- Record: found
- Abstract: found
- Article: not found