- Record: found
- Abstract: found
- Article: found
Wnt signaling in colorectal cancer: pathogenic role and therapeutic target

Read this article at
Abstract
Background
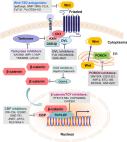
The Wnt signaling pathway is a complex network of protein interactions that functions most commonly in embryonic development and cancer, but is also involved in normal physiological processes in adults. The canonical Wnt signaling pathway regulates cell pluripotency and determines the differentiation fate of cells during development. The canonical Wnt signaling pathway (also known as the Wnt/β-catenin signaling pathway) is a recognized driver of colon cancer and one of the most representative signaling pathways. As a functional effector molecule of Wnt signaling, the modification and degradation of β-catenin are key events in the Wnt signaling pathway and the development and progression of colon cancer. Therefore, the Wnt signaling pathway plays an important role in the pathogenesis of diseases, especially the pathogenesis of colorectal cancer (CRC).
Objective
Inhibit the Wnt signaling pathway to explore the therapeutic targets of colorectal cancer.
Methods
Based on studying the Wnt pathway, master the biochemical processes related to the Wnt pathway, and analyze the relevant targets when drugs or inhibitors act on the Wnt pathway, to clarify the medication ideas of drugs or inhibitors for the treatment of diseases, especially colorectal cancer.
Results
Wnt signaling pathways include: Wnt/β-catenin or canonical Wnt signaling pathway, planar cell polarity (Wnt-PCP) pathway and Wnt-Ca 2+ signaling pathway. The Wnt signaling pathway is closely related to cancer cell proliferation, stemness, apoptosis, autophagy, metabolism, inflammation and immunization, microenvironment, resistance, ion channel, heterogeneity, EMT/migration/invasion/metastasis. Drugs/phytochemicals and molecular preparations for the Wnt pathway of CRC treatment have now been developed. Wnt inhibitors are also commonly used clinically for the treatment of CRC.
Related collections
Most cited references273
- Record: found
- Abstract: found
- Article: not found
Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities.

- Record: found
- Abstract: found
- Article: found
CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
