- Record: found
- Abstract: found
- Article: found
Activation of cGAS‐STING pathway – A possible cause of myofiber atrophy/necrosis in dermatomyositis and immune‐mediated necrotizing myopathy
Read this article at
Abstract
Objective
The objective was to investigate the expression of the cGAS‐STING pathway‐associated protein in idiopathic inflammatory myopathy (IIM) and to investigate whether it is related to myofiber atrophy/necrosis in patients with dermatomyositis and immune‐mediated necrotizing myopathy.
Material and Methods
Muscle specimens obtained by open biopsy from 26 IIM patients (14 with dermatomyositis (DM), 8 with immune‐mediated necrotizing myopathy (IMNM), and 4 with other types of IIM), 4 dystrophinopathy, and 9 control patients were assessed for expression of cGAS‐STING pathway members via Western blot, quantitative real‐time PCR analysis (qRT‐PCR), and immunochemistry. Meanwhile, analysis its location distribution througn immunochemistry.
Results
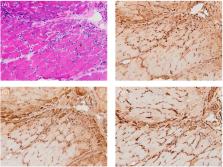
Compared to the control group, the expression of cGAS, STING, and related molecules was obviously increased in muscle samples of IIM patients. Upregulated cGAS and STING were mainly located in the vascular structure, inflammatory infiltrates, and atrophic and necrotic fibers. While comparing to the Dys patients, the mRNA level of cGAS, STING, and TNF‐a was upregulated, meanwhile, the protein of the TBK1, P‐TBK1, and P‐IRF3 associated with interferon upregulation was overexpressed through Western blot in IMNM and DM. Considering that cGAS and STING are located in necrotic and Mx1‐positive atrophic fibers, it is really possible that the cGAS‐STING pathway may lead to fibers atrophy/necrosis by producing IFNs.
Abstract
Related collections
Most cited references42

- Record: found
- Abstract: found
- Article: found
Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death
- Record: found
- Abstract: found
- Article: found
TDP-43 Triggers Mitochondrial DNA Release via mPTP to Activate cGAS/STING in ALS
- Record: found
- Abstract: not found
- Article: not found
