- Record: found
- Abstract: found
- Article: found
TDP-43 Triggers Mitochondrial DNA Release via mPTP to Activate cGAS/STING in ALS
Read this article at
Summary
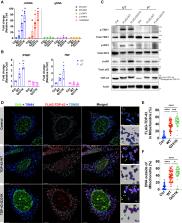
Cytoplasmic accumulation of TDP-43 is a disease hallmark for many cases of amyotrophic lateral sclerosis (ALS), associated with a neuroinflammatory cytokine profile related to upregulation of nuclear factor κB (NF-κB) and type I interferon (IFN) pathways. Here we show that this inflammation is driven by the cytoplasmic DNA sensor cyclic guanosine monophosphate (GMP)-AMP synthase (cGAS) when TDP-43 invades mitochondria and releases DNA via the permeability transition pore. Pharmacologic inhibition or genetic deletion of cGAS and its downstream signaling partner STING prevents upregulation of NF-κB and type I IFN induced by TDP-43 in induced pluripotent stem cell (iPSC)-derived motor neurons and in TDP-43 mutant mice. Finally, we document elevated levels of the specific cGAS signaling metabolite cGAMP in spinal cord samples from patients, which may be a biomarker of mtDNA release and cGAS/STING activation in ALS. Our results identify mtDNA release and cGAS/STING activation as critical determinants of TDP-43-associated pathology and demonstrate the potential for targeting this pathway in ALS.
Graphical Abstract
Highlights
-
•
TDP-43 enters mitochondria, triggers mtDNA release via the mPTP
-
•
TDP-43-induced cytosolic mtDNA accumulation activates the cGAS/STING pathway
-
•
Evidence of cytoplasmic mtDNA was found in ALS patient cells and disease models
-
•
Blocking STING prevents inflammation and neurodegeneration in vitro and in vivo
Abstract
TDP-43 causes inflammation in ALS by stimulating mitochondrial DNA release, which is subsequently sensed by the cytosolic cGAS/STING pathway, suggesting that inhibition of cGAS/STING could help alleviate inflammation-related damage in ALS.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
A role for mitochondria in NLRP3 inflammasome activation.
- Record: found
- Abstract: found
- Article: not found
Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis.
- Record: found
- Abstract: found
- Article: not found
