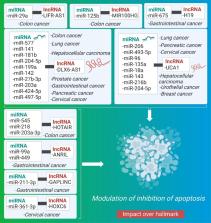
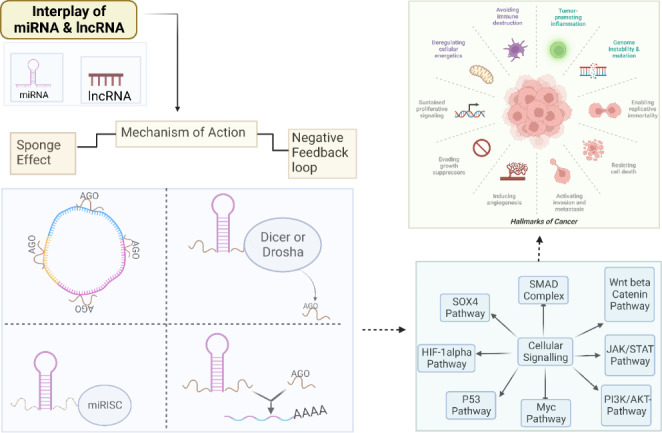
[1
]GRID grid.412122.6, ISNI 0000 0004 1808 2016, KIIT School of Biotechnology, , Kalinga Institute of Industrial Technology (KIIT-DU), ; Bhubaneswar, Odisha India
[2
]GRID grid.9026.d, ISNI 0000 0001 2287 2617, Present Address: Centre for Structural System Biology, Department of Physics, , University of Hamburg, ; c/o DESY, Building 15, Notkestr. 852267, Hamburg, Germany
[3
]GRID grid.462378.c, ISNI 0000 0004 1764 2464, School of Biology, , Indian Institute of Science Education and Research, ; Thiruvananthapuram, India
[4
]GRID grid.444644.2, ISNI 0000 0004 1805 0217, Amity Institute of Biotechnology, , Amity University Jharkhand, ; Ranchi, Jharkhand, 834001 India
[5
]GRID grid.444415.4, ISNI 0000 0004 1759 0860, School of Health Sciences and Technology (SoHST), , UPES University, ; Dehradun, Uttarakhand India
[6
]GRID grid.5373.2, ISNI 0000000108389418, Department of Applied Physics, School of Science, , Aalto University, ; Espoo, 00076 Finland
[7
]GRID grid.5373.2, ISNI 0000000108389418, Department of Bioproducts and Biosystems, School of Chemical Engineering, , Aalto University, ; Espoo, 00076 Finland
[8
]GRID grid.412552.5, ISNI 0000 0004 1764 278X, Department of Biotechnology, School of Engineering and Technology (SET), , Sharda University, ; Greater Noida, 201310 UP India
[9
]GRID grid.449005.c, School of Bioengineering & Biosciences, , Lovely Professional University, ; Phagwara, 144411 India
[10
]GRID grid.449906.6, ISNI 0000 0004 4659 5193, Department of Biotechnology, School of Applied & Life Sciences (SALS), , Uttaranchal University, ; Dehradun, 248007 India
[11
]GRID grid.7737.4, ISNI 0000 0004 0410 2071, Faculty of Biological and Environmental Sciences, , University of Helsinki, ; Biocentre 3, Helsinki, Finland
[12
]GRID grid.13797.3b, ISNI 0000 0001 2235 8415, Pharmacy, , Abo Akademi University, ; Tykistökatu 6A, Turku, Finland
License:
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License,
which permits use, sharing, adaptation, distribution and reproduction in any medium
or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons licence, and indicate if changes were
made. The images or other third party material in this article are included in the
article’s Creative Commons licence, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons licence
and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view
a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/.