- Record: found
- Abstract: found
- Article: found
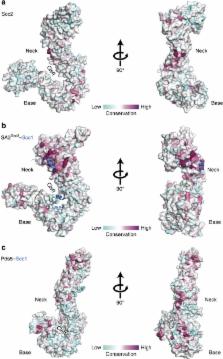
Structure of the cohesin loader Scc2
Read this article at
Abstract
The functions of cohesin are central to genome integrity, chromosome organization and transcription regulation through its prevention of premature sister-chromatid separation and the formation of DNA loops. The loading of cohesin onto chromatin depends on the Scc2–Scc4 complex; however, little is known about how it stimulates the cohesion-loading activity. Here we determine the large ‘hook' structure of Scc2 responsible for catalysing cohesin loading. We identify key Scc2 surfaces that are crucial for cohesin loading in vivo. With the aid of previously determined structures and homology modelling, we derive a pseudo-atomic structure of the full-length Scc2–Scc4 complex. Finally, using recombinantly purified Scc2–Scc4 and cohesin, we performed crosslinking mass spectrometry and interaction assays that suggest Scc2–Scc4 uses its modular structure to make multiple contacts with cohesin.
Abstract
The cohesin complex maintains genome integrity by ensuring correct sister-chromatid segregation during mitosis and meiosis. Here, Chao et al. present a pseudo-atomic model of the full-length Scc2–Scc4 cohesin loader complex and reveal key Scc2 surfaces crucial for cohesin loading.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Cohesins: chromosomal proteins that prevent premature separation of sister chromatids.
- Record: found
- Abstract: found
- Article: not found
Cohesin's binding to chromosomes depends on a separate complex consisting of Scc2 and Scc4 proteins.
- Record: found
- Abstract: found
- Article: not found