- Record: found
- Abstract: found
- Article: found
Sulforaphane Attenuates Chronic Intermittent Hypoxia-Induced Brain Damage in Mice via Augmenting Nrf2 Nuclear Translocation and Autophagy
Read this article at
Abstract
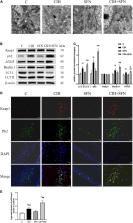
Obstructive sleep apnea–hypopnea syndrome (OSAHS), typically characterized by chronic intermittent hypoxia (CIH), is associated with neurocognitive dysfunction in children. Sulforaphane (SFN), an activator of nuclear factor E2-related factor 2 (Nrf2), has been demonstrated to protect against oxidative stress in various diseases. However, the effect of SFN on OSAHS remains elusive. In this research, we investigated the neuroprotective role of SFN in CIH-induced cognitive dysfunction and underlying mechanisms of regulation of Nrf2 signaling pathway and autophagy. CIH exposures for 4 weeks in mice, modeling OSAHS, contributed to neurocognitive dysfunction, manifested as increased working memory errors (WMEs), reference memory errors (RMEs) and total memory errors (TEs) in the 8-arm radial maze test. The mice were intraperitoneally injected with SFN (0.5 mg/kg) 30 min before CIH exposure everyday. SFN treatment ameliorated neurocognitive dysfunction in CIH mice, which demonstrates less RME, WME, and TE. Also, SFN effectively alleviated apoptosis of hippocampal neurons following CIH by decreased TUNEL-positive cells, downregulated cleaved PARP, cleaved caspase 3, and upregulated Bcl-2. SFN protects hippocampal tissue from CIH-induced oxidative stress as evidenced by elevated superoxide dismutase (SOD) activities and reduced malondialdehyde (MDA). In addition, we found that SFN enhanced Nrf2 nuclear translocation to hold an antioxidative function on CIH-induced neuronal apoptosis in hippocampus. Meanwhile, SFN promoted autophagy activation, as shown by increased Beclin1, ATG5, and LC3II/LC3I. Overall, our findings indicated that SFN reduced the apoptosis of hippocampal neurons through antioxidant effect of Nrf2 and autophagy in CIH-induced brain damage, which highlights the potential of SFN as a novel therapy for OSAHS-related neurocognitive dysfunction.
Related collections
Most cited references39

- Record: found
- Abstract: found
- Article: found
Oxidative stress and autophagy: the clash between damage and metabolic needs
- Record: found
- Abstract: found
- Article: not found
p62 links autophagy and Nrf2 signaling.
- Record: found
- Abstract: found
- Article: not found