- Record: found
- Abstract: found
- Article: found
Human Rap1 modulates TRF2 attraction to telomeric DNA
Read this article at
Abstract
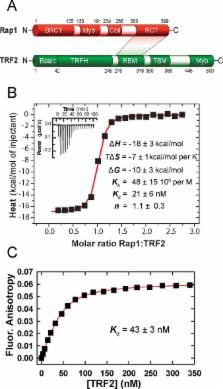
More than two decades of genetic research have identified and assigned main biological functions of shelterin proteins that safeguard telomeres. However, a molecular mechanism of how each protein subunit contributes to the protecting function of the whole shelterin complex remains elusive. Human Repressor activator protein 1 (Rap1) forms a multifunctional complex with Telomeric Repeat binding Factor 2 (TRF2). Rap1–TRF2 complex is a critical part of shelterin as it suppresses homology-directed repair in Ku 70/80 heterodimer absence. To understand how Rap1 affects key functions of TRF2, we investigated full-length Rap1 binding to TRF2 and Rap1–TRF2 complex interactions with double-stranded DNA by quantitative biochemical approaches. We observed that Rap1 reduces the overall DNA duplex binding affinity of TRF2 but increases the selectivity of TRF2 to telomeric DNA. Additionally, we observed that Rap1 induces a partial release of TRF2 from DNA duplex. The improved TRF2 selectivity to telomeric DNA is caused by less pronounced electrostatic attractions between TRF2 and DNA in Rap1 presence. Thus, Rap1 prompts more accurate and selective TRF2 recognition of telomeric DNA and TRF2 localization on single/double-strand DNA junctions. These quantitative functional studies contribute to the understanding of the selective recognition of telomeric DNA by the whole shelterin complex.
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: not found
Homologous recombination generates T-loop-sized deletions at human telomeres.
- Record: found
- Abstract: found
- Article: not found
T-loop assembly in vitro involves binding of TRF2 near the 3' telomeric overhang.
- Record: found
- Abstract: found
- Article: not found