- Record: found
- Abstract: found
- Article: found
Early and Next-Generation KIT/PDGFRA Kinase Inhibitors and the Future of Treatment for Advanced Gastrointestinal Stromal Tumor
Read this article at
Abstract
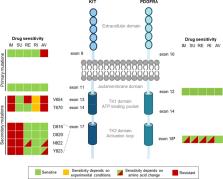
The majority of gastrointestinal stromal tumors (GIST) harbor an activating mutation in either the KIT or PDGFRA receptor tyrosine kinases. Approval of imatinib, a KIT/PDGFRA tyrosine kinase inhibitor (TKI), meaningfully improved the treatment of advanced GIST. Other TKIs subsequently gained approval: sunitinib as a second-line therapy and regorafenib as a third-line therapy. However, resistance to each agent occurs in almost all patients over time, typically due to secondary kinase mutations. A major limitation of these 3 approved therapies is that they target the inactive conformation of KIT/PDGFRA; thus, their efficacy is blunted against secondary mutations in the kinase activation loop. Neither sunitinib nor regorafenib inhibit the full spectrum of KIT resistance mutations, and resistance is further complicated by extensive clonal heterogeneity, even within single patients. To combat these limitations, next-generation TKIs were developed and clinically tested, leading to 2 new USA FDA drug approvals in 2020. Ripretinib, a broad-spectrum KIT/PDGFRA inhibitor, was recently approved for the treatment of adult patients with advanced GIST who have received prior treatment with 3 or more kinase inhibitors, including imatinib. Avapritinib, a type I kinase inhibitor that targets active conformation, was approved for the treatment of adults with unresectable or metastatic GIST harboring a PDGFRA exon 18 mutation, including PDGFRA D842V mutations. In this review, we will discuss how resistance mutations have driven the need for newer treatment options for GIST and compare the original GIST TKIs with the next-generation KIT/PDGFRA kinase inhibitors, ripretinib and avapritinib, with a focus on their mechanisms of action.
Related collections
Most cited references82
- Record: found
- Abstract: found
- Article: not found
PDGFRA activating mutations in gastrointestinal stromal tumors.
- Record: found
- Abstract: found
- Article: not found
Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial.
- Record: found
- Abstract: found
- Article: not found