- Record: found
- Abstract: found
- Article: found
Anogenital Distance Plasticity in Adulthood: Implications for Its Use as a Biomarker of Fetal Androgen Action
Read this article at
Abstract
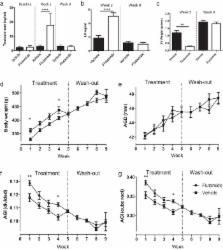
Androgen action during the fetal masculinization programming window (MPW) determines the maximum potential for growth of androgen-dependent organs (eg, seminal vesicles, prostate, penis, and perineum) and is reflected in anogenital distance (AGD). As such, determining AGD in postnatal life has potential as a lifelong easily accessible biomarker of overall androgen action during the MPW. However, whether the perineum remains androgen responsive in adulthood and thus responds plastically to perturbed androgen drive remains unexplored. To determine this, we treated adult male rats with either the antiandrogen flutamide or the estrogen diethylstilbestrol (DES) for 5 weeks, followed by a 4-week washout period of no treatment. We determined AGD and its correlate anogenital index (AGI) (AGD relative to body weight) at weekly intervals across this period and compared these with normal adult rats (male and female), castrated male rats, and appropriate vehicle controls. These data showed that, in addition to reducing circulating testosterone and seminal vesicle weight, castration significantly reduced AGD (by ∼17%), demonstrating that there is a degree of plasticity in AGD in adulthood. Flutamide treatment increased circulating testosterone yet also reduced seminal vesicle weight due to local antagonism of androgen receptor. Despite this suppression, surprisingly, flutamide treatment had no effect on AGD at any time point. In contrast, although DES treatment suppressed circulating testosterone and reduced seminal vesicle weight, it also induced a significant reduction in AGD (by ∼11%), which returned to normal 1 week after cessation of DES treatment. We conclude that AGD in adult rats exhibits a degree of plasticity, which may be mediated by modulation of local androgen/estrogen action. The implications of these findings regarding the use of AGD as a lifelong clinical biomarker of fetal androgen action are discussed.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism.
- Record: found
- Abstract: found
- Article: not found
Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice.
- Record: found
- Abstract: found
- Article: not found