- Record: found
- Abstract: found
- Article: found
Ablation of the canonical testosterone production pathway via knockout of the steroidogenic enzyme HSD17B3, reveals a novel mechanism of testicular testosterone production
Read this article at
Abstract
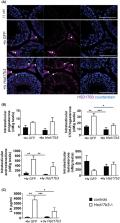
Male development, fertility, and lifelong health are all androgen‐dependent. Approximately 95% of circulating testosterone is synthesized by the testis and the final step in this canonical pathway is controlled by the activity of the hydroxysteroid‐dehydrogenase‐17‐beta‐3 (HSD17B3). To determine the role of HSD17B3 in testosterone production and androgenization during male development and function we have characterized a mouse model lacking HSD17B3. The data reveal that developmental masculinization and fertility are normal in mutant males. Ablation of HSD17B3 inhibits hyperstimulation of testosterone production by hCG, although basal testosterone levels are maintained despite the absence of HSD17B3. Reintroduction of HSD17B3 via gene‐delivery to Sertoli cells in adulthood partially rescues the adult phenotype, showing that, as in development, different cell‐types in the testis are able to work together to produce testosterone. Together, these data show that HS17B3 acts as a rate‐limiting‐step for the maximum level of testosterone production by the testis but does not control basal testosterone production. Measurement of other enzymes able to convert androstenedione to testosterone identifies HSD17B12 as a candidate enzyme capable of driving basal testosterone production in the testis. Together, these findings expand our understanding of testosterone production in males.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism.
- Record: found
- Abstract: found
- Article: not found
Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study.
- Record: found
- Abstract: found
- Article: not found