- Record: found
- Abstract: found
- Article: found
Spatiotemporal Control of Intracellular Membrane Trafficking by Rho GTPases
Read this article at
Abstract
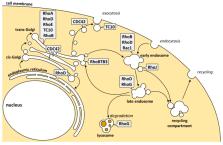
As membrane-associated master regulators of cytoskeletal remodeling, Rho GTPases coordinate a wide range of biological processes such as cell adhesion, motility, and polarity. In the last years, Rho GTPases have also been recognized to control intracellular membrane sorting and trafficking steps directly; however, how Rho GTPase signaling is regulated at endomembranes is still poorly understood. In this review, we will specifically address the local Rho GTPase pools coordinating intracellular membrane trafficking with a focus on the endo- and exocytic pathways. We will further highlight the spatiotemporal molecular regulation of Rho signaling at endomembrane sites through Rho regulatory proteins, the GEFs and GAPs. Finally, we will discuss the contribution of dysregulated Rho signaling emanating from endomembranes to the development and progression of cancer.
Related collections
Most cited references153
- Record: found
- Abstract: found
- Article: not found
Membrane recognition by phospholipid-binding domains.
- Record: found
- Abstract: found
- Article: not found