- Record: found
- Abstract: found
- Article: found
Platelet Function and Therapeutic Applications in Dogs: Current Status and Future Prospects
Read this article at
Abstract
Simple Summary
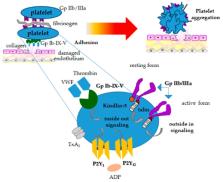
Platelets, small blood cells produced from megakaryocytes mainly in the bone marrow, are implicated not only in hemostasis, but also in different physiological and pathophysiological processes. This paper reviews canine platelet structure and functions, including an accurate description of the numerous surface receptors involved in the mechanisms of platelet adhesion and aggregation. In addition, we describe the most important canine platelet disorders that can affect platelet numbers (thrombocytopenias and thrombocytosis) or platelet function (thrombopathias). Platelet-related tests are vital in the analysis of primary hemostatic disorders and for this reason, we also discuss the efficacy of various tests in the diagnosis of canine platelet disorders. Finally, innovative therapeutic approaches based on the use of platelets and their derivatives for the treatment of various canine diseases such as inflammatory conditions (tendinitis and osteoarthritis) and for wound healing and tissue repair are reported.
Abstract
Significant progress has been made in the functional characterization of canine platelets in the last two decades. The role of canine platelets in hemostasis includes their adhesion to the subendothelium, activation, and aggregation, leading to primary clot formation at the site of injury. Studies on canine platelet function and advancements in laboratory testing have improved the diagnosis and understanding of platelet-related disorders as well as the knowledge of the mechanisms behind these diseases. This review focuses on the most recent discoveries in canine platelet structure, function, and disorders; and discusses the efficacy of various tests in the diagnosis of platelet-related disorders. With the relatively recent discovery of angiogenetic and reparative effects of growth factors found in platelets, this review also summarizes the use of canine platelet-rich plasma (PRP) alone or in association with stem cells in regenerative therapy. The characterization of proteomic and lipidomic profiles and development of platelet gene therapy in veterinary species are areas of future study with potential for major therapeutic benefits.
Related collections
Most cited references178
- Record: found
- Abstract: found
- Article: not found
Platelet-rich plasma: a milieu of bioactive factors.
- Record: found
- Abstract: found
- Article: not found
The platelet release reaction: granules' constituents, secretion and functions.
- Record: found
- Abstract: found
- Article: not found