- Record: found
- Abstract: found
- Article: found
Nociceptors as chronic drivers of pain and hyperreflexia after spinal cord injury: an adaptive-maladaptive hyperfunctional state hypothesis
Read this article at
Abstract
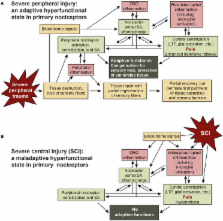
Spinal cord injury (SCI) causes chronic peripheral sensitization of nociceptors and persistent generation of spontaneous action potentials (SA) in peripheral branches and the somata of hyperexcitable nociceptors within dorsal root ganglia (DRG). Here it is proposed that SCI triggers in numerous nociceptors a persistent hyperfunctional state (peripheral, synaptic, and somal) that originally evolved as an adaptive response to compensate for loss of sensory terminals after severe but survivable peripheral injury. In this hypothesis, nociceptor somata monitor the status of their own receptive field and the rest of the body by integrating signals received by their peripheral and central branches and the soma itself. A nociceptor switches into a potentially permanent hyperfunctional state when central neural, glial, and inflammatory signal combinations are detected that indicate extensive peripheral injury. Similar signal combinations are produced by SCI and disseminated widely to uninjured as well as injured nociceptors. This paper focuses on the uninjured nociceptors that are altered by SCI. Enhanced activity generated in below-level nociceptors promotes below-level central sensitization, somatic and autonomic hyperreflexia, and visceral dysfunction. If sufficient ascending fibers survive, enhanced activity in below-level nociceptors contributes to below-level pain. Nociceptor activity generated above the injury level contributes to at- and above-level sensitization and pain (evoked and spontaneous). Thus, SCI triggers a potent nociceptor state that may have been adaptive (from an evolutionary perspective) after severe peripheral injury but is maladaptive after SCI. Evidence that hyperfunctional nociceptors make large contributions to behavioral hypersensitivity after SCI suggests that nociceptor-specific ion channels required for nociceptor SA and hypersensitivity offer promising targets for treating chronic pain and hyperreflexia after SCI.
Related collections
Most cited references148
- Record: found
- Abstract: found
- Article: not found
The molecular biology of memory storage: a dialogue between genes and synapses.
- Record: found
- Abstract: found
- Article: not found
Transient receptor potential channels: targeting pain at the source.
- Record: found
- Abstract: found
- Article: not found