- Record: found
- Abstract: found
- Article: found
Apelin Ameliorates TNF-α-Induced Reduction of Glycogen Synthesis in the Hepatocytes through G Protein-Coupled Receptor APJ
Read this article at
Abstract
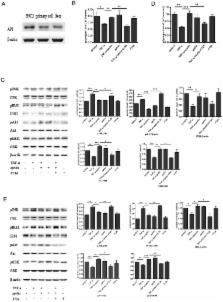
Apelin, a novel adipokine, is the specific endogenous ligand of G protein-coupled receptor APJ. Consistent with its putative role as an adipokine, apelin has been linked to states of insulin resistance. However, the function of apelin in hepatic insulin resistance, a vital part of insulin resistance, and its underlying mechanisms still remains unclear. Here we define the impacts of apelin on TNF-α-induced reduction of glycogen synthesis in the hepatocytes. Our studies indicate that apelin reversed TNF-α-induced reduction of glycogen synthesis in HepG2 cells, mouse primary hepatocytes and liver tissues of C57BL/6J mice by improving JNK-IRS1-AKT-GSK pathway. Moreover, Western blot revealed that APJ, but not apelin, expressed in the hepatocytes and liver tissues of mice. We found that F13A, a competitive antagonist for G protein-coupled receptor APJ, suppressed the effects of apelin on TNF-α-induced reduction of glycogen synthesis in the hepatocytes, suggesting APJ is involved in the function of apelin. In conclusion, we show novel evidence suggesting that apelin ameliorates TNF-α-induced reduction of glycogen synthesis in the hepatocytes through G protein-coupled receptor APJ. Apelin appears as a beneficial adipokine with anti-insulin resistance properties, and thus as a promising therapeutic target in metabolic disorders.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Adipocytes as regulators of energy balance and glucose homeostasis.
- Record: found
- Abstract: not found
- Article: not found
Preparation of isolated rat liver cells.
- Record: found
- Abstract: found
- Article: not found