- Record: found
- Abstract: found
- Article: found
The chemokine receptor CCR10 promotes inflammation-driven hepatocarcinogenesis via PI3K/Akt pathway activation

Read this article at
Abstract
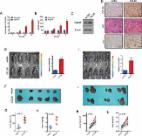
G-protein-coupled receptor (GPCR)-related proteins are dysregulated and the GPCR CC-chemokine receptor 10 (CCR10) is significantly upregulated in inflammation-driven HCC. However, CCR10′s role in inflammation-driven hepatocarcinogenesis remains unknown. The aim of this study was to evaluate the role of CCR10 in inflammation-driven hepatocarcinogenesis. Via a targeted gene expression microarray screening alterations in GPCR family gene expression, we found CCR10 to be significantly upregulated in hepatocytes isolated from inflammation-driven human HCC tumors and matching paracancerous tissues. Tetrachloromethane (CCl4)-induced and diethylnitrosamine (DEN)-induced murine models of inflammatory hepatocarcinogenesis displayed significant hepatocellular TNF and CCR10 upregulation. Exogenous TNF applied to HepG2 and LO2 cell lines as well as wild-type (WT) mice significantly upregulated hepatocellular CCR10 expression, Akt phosphorylation, PCNA expression, and hepatocellular proliferation. Additionally, exogenous TNF significantly upregulated secretion of the natural CCR10 ligand-agonist CCL28 from both cell lines. Transgenic CCR10-knockout (CCR10 KO) in DEN-treated mice significantly increased hepatocellular apoptosis levels and significantly lowered compensatory hepatocellular proliferation but did not affect upstream TNF expression. In addition, DEN-treated CCR10 KO mice showed a significantly lower liver weight/body weight ratio, significantly lower liver tumor incidence, and significantly smaller tumors. Moreover, exogenous CCR10 expression significantly raised xenograft tumor growth in Balb/c nude mice. In vitro, CCR10 transfection or CCL28 treatment in HepG2 and LO2 cell lines significantly increased Akt phosphorylation, PCNA expression, and cell proliferation, while CCR10 silencing or Akt inhibition produced the opposite effects. In vivo, hepatocytes isolated from HCC tumor tissue and matching paracancerous tissue in DEN-treated CCR10 KO mice showed significantly lower Akt phosphorylation and PCNA expression relative to WT hepatocytes. In conclusion, inflammation-induced TNF promotes hepatocellular CCR10 expression and downstream PI3K/Akt-mediated hepatocarcinogenesis. CCR10 appears to function as a linkage between TNF stimulation and downstream PI3K/Akt pathway activation and shows promise as a potential therapeutic target for inflammation-driven HCC.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Obesity, inflammation, and liver cancer.
- Record: found
- Abstract: found
- Article: not found
DCs metabolize sunlight-induced vitamin D3 to 'program' T cell attraction to the epidermal chemokine CCL27.
- Record: found
- Abstract: found
- Article: found