- Record: found
- Abstract: found
- Article: found
Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria
Read this article at
Abstract
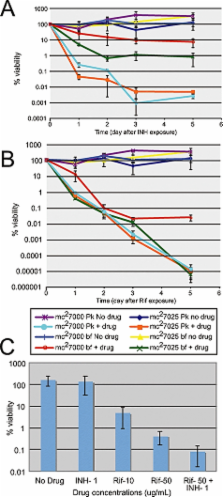
Successful treatment of human tuberculosis requires 6–9 months' therapy with multiple antibiotics. Incomplete clearance of tubercle bacilli frequently results in disease relapse, presumably as a result of reactivation of persistent drug-tolerant Mycobacterium tuberculosis cells, although the nature and location of these persisters are not known. In other pathogens, antibiotic tolerance is often associated with the formation of biofilms – organized communities of surface-attached cells – but physiologically and genetically defined M. tuberculosis biofilms have not been described. Here, we show that M. tuberculosis forms biofilms with specific environmental and genetic requirements distinct from those for planktonic growth, which contain an extracellular matrix rich in free mycolic acids, and harbour an important drug-tolerant population that persist despite exposure to high levels of antibiotics.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Bacterial persistence as a phenotypic switch.
- Record: found
- Abstract: found
- Article: not found
Persister cells, dormancy and infectious disease.
- Record: found
- Abstract: found
- Article: not found