- Record: found
- Abstract: found
- Article: found
Trial in Elderly with Musculoskeletal Problems due to Underlying Sarcopenia—Faeces to Unravel the Gut and Inflammation Translationally (TEMPUS-FUGIT): protocol of a cross-sequential study to explore the gut-muscle axis in the development and treatment of sarcopenia in community-dwelling older adults
Read this article at
Abstract
Background
Gut microbiota (GM) might play a role in muscle metabolism and physiological processes through a hypothesized gut-muscle axis, influencing muscle mass and function and thus, sarcopenia. The Trial in Elderly with Musculoskeletal Problems due to Underlying Sarcopenia—Faeces to Unravel the Gut and Inflammation Translationally (TEMPUS-FUGIT) aims to explore the gut-muscle axis in sarcopenia.
Methods
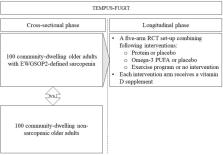
First, in a cross-sectional case–control phase, 100 community-dwelling adults without sarcopenia will be compared to 100 community-dwelling adults (≥ 65 years) with sarcopenia of similar age-, gender and BMI-ratio, participating in the ongoing ‘Exercise and Nutrition for Healthy AgeiNg’ (ENHANce; NCT03649698) study. Sarcopenia is diagnosed according to the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) criteria. GM composition and intestinal inflammatory markers (fecal calprotectin, lactoferrin and S100A12) will be determined in fecal samples. Systemic inflammatory markers (hs-CRP, IL-4, IL-6, TNF-α, IL-13, IL-1β and creatine kinase) will be determined in fasted blood samples. Both groups will be compared using appropriate statistical testing, whereas linear regression will be used for cross-sectional associations between gut, inflammatory and sarcopenia parameters.
Second, in the longitudinal phase, sarcopenic older adults will be requested to deliver five fecal samples during the 12-week intervention to assess the effects of protein, omega-3 and a physical exercise program on the GM.
Discussion
TEMPUS-FUGIT aims to explore the gut-muscle axis by comparing GM composition between sarcopenic and non-sarcopenic older adults and to determine the association of GM with intestinal and systemic inflammatory markers and sarcopenia-defining parameters (muscle mass, muscle strength and physical performance). Furthermore, effects of single or combined, optimized and individualized anabolic interventions (exercise, protein and omega-3 supplementation), on GM will be explored in persons with sarcopenia.
TEMPUS-FUGIT aims to impact clinical practice by clarifying the relationship between the gut-muscle axis and sarcopenia. TEMPUS-FUGIT is expected to contribute to the discovery of clinical and microbial biomarkers for sarcopenia and insights in its pathophysiology, opening possible future perspectives for novel sarcopenia treatment strategies targeting GM.
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: not found
An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies
- Record: found
- Abstract: found
- Article: not found
SPIRIT 2013 statement: defining standard protocol items for clinical trials.
- Record: found
- Abstract: found
- Article: not found