- Record: found
- Abstract: found
- Article: found
Biomaterials with Antibacterial and Osteoinductive Properties to Repair Infected Bone Defects
Read this article at
Abstract
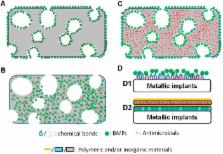
The repair of infected bone defects is still challenging in the fields of orthopedics, oral implantology and maxillofacial surgery. In these cases, the self-healing capacity of bone tissue can be significantly compromised by the large size of bone defects and the potential/active bacterial activity. Infected bone defects are conventionally treated by a systemic/local administration of antibiotics to control infection and a subsequent implantation of bone grafts, such as autografts and allografts. However, these treatment options are time-consuming and usually yield less optimal efficacy. To approach these problems, novel biomaterials with both antibacterial and osteoinductive properties have been developed. The antibacterial property can be conferred by antibiotics and other novel antibacterial biomaterials, such as silver nanoparticles. Bone morphogenetic proteins are used to functionalize the biomaterials with a potent osteoinductive property. By manipulating the carrying modes and release kinetics, these biomaterials are optimized to maximize their antibacterial and osteoinductive functions with minimized cytotoxicity. The findings, in the past decade, have shown a very promising application potential of the novel biomaterials with the dual functions in treating infected bone defects. In this review, we will summarize the current knowledge of novel biomaterials with both antibacterial and osteoinductive properties.
Related collections
Most cited references111
- Record: found
- Abstract: found
- Article: not found
Antimicrobial properties of chitosan and mode of action: a state of the art review.
- Record: found
- Abstract: found
- Article: not found
Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species.
- Record: found
- Abstract: found
- Article: not found