- Record: found
- Abstract: found
- Article: found
Individualised Approaches for Catheter Ablation of AF: Patient Selection and Procedural Endpoints
Read this article at
Abstract
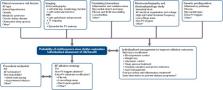
Pulmonary vein isolation (PVI) is the cornerstone of AF ablation, but studies have reported improved efficacy with high rates of repeat procedures. Because of the large interindividual variability in the underlying electrical and anatomical substrate, achieving optimal outcomes requires an individualised approach. This includes optimal candidate selection as well as defined ablation strategies with objective procedure endpoints beyond PVI. Candidate selection is traditionally based on coarse and sometimes arbitrary clinical stratification such as AF type, but finer predictors of treatment efficacy including biomarkers, advanced imaging and electrocardiographic parameters have shown promise. Numerous ancillary ablation strategies beyond PVI have been investigated, but the absence of a clear mechanistic and evidence-based endpoint, unlike in other arrhythmias, has remained a universal limitation. Potential endpoints include functional ones such as AF termination or non-inducibility and substrate-based endpoints such as isolation of low-voltage areas. This review summarises the relevant literature and proposes guidance for clinical practice and future research.
Related collections
Most cited references68
- Record: found
- Abstract: found
- Article: not found
Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats.
- Record: found
- Abstract: found
- Article: not found
Electrical, contractile and structural remodeling during atrial fibrillation.
- Record: found
- Abstract: found
- Article: not found