- Record: found
- Abstract: found
- Article: found
N,P-Codoped Carbon Layer Coupled with MoP Nanoparticles as an Efficient Electrocatalyst for Hydrogen Evolution Reaction
research-article
30 July 2018
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
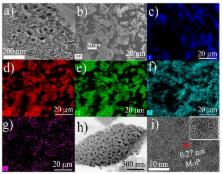
Efficient electrocatalyst plays a significant role on the development of hydrogen energy. In this work, an N,P-codoped carbon layer coupled with MoP nanoparticles (MoP/NPCs) was prepared through a facile high-temperature pyrolysis treatment. The obtained MoP/NPCs presented efficient activity for hydrogen evolution reaction (HER), with low onset potential of 90 mV, and a small Tafel slope (71 mV dec −1), as well as extraordinary stability in acidic electrolyte. This work provides a new facile strategy for the design and synthesis of sustainable and effective molybdenum-based electrocatalysts as alternatives to non-Pt catalysts for HER.
Related collections
Most cited references36
- Record: found
- Abstract: not found
- Article: not found
Tailoring the d-Band Centers Enables Co4 N Nanosheets To Be Highly Active for Hydrogen Evolution Catalysis
Zhiyan Chen, Yao Song, Jinyan Cai … (2018)
- Record: found
- Abstract: not found
- Article: not found
Multifunctional Carbon-Based Metal-Free Electrocatalysts for Simultaneous Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution
Liming Dai, Chuangang Hu (2017)
- Record: found
- Abstract: found
- Article: not found
Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C
Yuan-Yuan Ma, Cai-Xia Wu, Xiao-Jia Feng … (2017)