- Record: found
- Abstract: found
- Article: not found
Prospective Randomized Study of Doxorubicin-Eluting-Bead Embolization in the Treatment of Hepatocellular Carcinoma: Results of the PRECISION V Study
Read this article at
Abstract
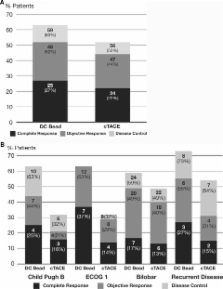
Transcatheter arterial chemoembolization (TACE) offers a survival benefit to patients with intermediate hepatocellular carcinoma (HCC). A widely accepted TACE regimen includes administration of doxorubicin-oil emulsion followed by gelatine sponge—conventional TACE. Recently, a drug-eluting bead (DC Bead ®) has been developed to enhance tumor drug delivery and reduce systemic availability. This randomized trial compares conventional TACE (cTACE) with TACE with DC Bead for the treatment of cirrhotic patients with HCC. Two hundred twelve patients with Child-Pugh A/B cirrhosis and large and/or multinodular, unresectable, N0, M0 HCCs were randomized to receive TACE with DC Bead loaded with doxorubicin or cTACE with doxorubicin. Randomization was stratified according to Child-Pugh status (A/B), performance status (ECOG 0/1), bilobar disease (yes/no), and prior curative treatment (yes/no). The primary endpoint was tumor response (EASL) at 6 months following independent, blinded review of MRI studies. The drug-eluting bead group showed higher rates of complete response, objective response, and disease control compared with the cTACE group (27% vs. 22%, 52% vs. 44%, and 63% vs. 52%, respectively). The hypothesis of superiority was not met (one-sided P = 0.11). However, patients with Child-Pugh B, ECOG 1, bilobar disease, and recurrent disease showed a significant increase in objective response ( P = 0.038) compared to cTACE. DC Bead was associated with improved tolerability, with a significant reduction in serious liver toxicity ( P < 0.001) and a significantly lower rate of doxorubicin-related side effects ( P = 0.0001). TACE with DC Bead and doxorubicin is safe and effective in the treatment of HCC and offers a benefit to patients with more advanced disease.
Related collections
Most cited references24
- Record: found
- Abstract: not found
- Article: not found
Management of hepatocellular carcinoma.
- Record: found
- Abstract: not found
- Article: not found
Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver.
- Record: found
- Abstract: found
- Article: not found
