- Record: found
- Abstract: found
- Article: found
Oncolytic virotherapy evolved into the fourth generation as tumor immunotherapy
Read this article at
Abstract
Background
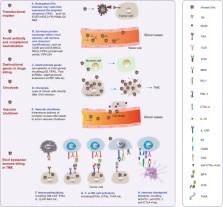
Oncolytic virotherapy (OVT) is a promising anti-tumor modality that utilizes oncolytic viruses (OVs) to preferentially attack cancers rather than normal tissues. With the understanding particularly in the characteristics of viruses and tumor cells, numerous innovative OVs have been engineered to conquer cancers, such as Talimogene Laherparepvec (T-VEC) and tasadenoturev (DNX-2401). However, the therapeutic safety and efficacy must be further optimized and balanced to ensure the superior safe and efficient OVT in clinics, and reasonable combination therapy strategies are also important challenges worthy to be explored.
Main body
Here we provided a critical review of the development history and status of OVT, emphasizing the mechanisms of enhancing both safety and efficacy. We propose that oncolytic virotherapy has evolved into the fourth generation as tumor immunotherapy. Particularly, to arouse T cells by designing OVs expressing bi-specific T cell activator (BiTA) is a promising strategy of killing two birds with one stone. Amazing combination of therapeutic strategies of OVs and immune cells confers immense potential for managing cancers. Moreover, the attractive preclinical OVT addressed recently, and the OVT in clinical trials were systematically reviewed.
Related collections
Most cited references183
- Record: found
- Abstract: found
- Article: not found
Cancer Statistics, 2021
- Record: found
- Abstract: found
- Article: not found
Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma.
- Record: found
- Abstract: found
- Article: not found