- Record: found
- Abstract: found
- Article: found
Quality of a Supporting Mobile App for Rheumatic Patients: Patient-Based Assessment Using the User Version of the Mobile Application Scale (uMARS)

Read this article at
Abstract
Introduction: Mobile applications promise to improve current health care. However, current mobile app quality ratings are mostly physician-based. The aim of this study was (1) to assess the quality of the self-management app Rheuma Auszeit using the validated uMARS (User Version of the Mobile App Rating Scale) app quality assessment tool and (2) to evaluate the association between uMARS scores and patients' characteristics.
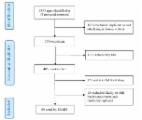
Materials and Methods: Consecutive patients with rheumatoid arthritis, psoriatic arthritis and spondyloarthritis were seen at the rheumatology clinic at university hospital Erlangen, Germany. They were asked to test Rheuma Auszeit, evaluate its quality using uMARS and complete a paper-based survey evaluating the individual preferences, attitudes and ehealth literacy. The association between uMARS scores and patients' characteristics was further explored.
Results: Between December 2018 and January 2019, a total of 126 patients evaluated Rheuma Auszeit using uMARS and filled out the paper-based survey. The median uMARS score was 3.9, IQR 0.7. Functionality was the domain with the highest rating (median 4.8, IQR 0.8), followed by aesthetics (median 4.0, IQR 0.7), information (median 3.5, IQR 0.8), and engagement (median 3.2, IQR 1.0). Subjective quality was average (median 3.0, IQR 1.0). The lowest scoring individual item was customization with a median of 2.5/5. Lower functionality scores were reported among older female rheumatic patients ( P < 0.004). Older male rheumatic patients reported a higher subjective quality score ( P < 0.024). Perceived disease activity and disease duration did not significantly correlate with any uMARS subdomain scores. eHealth literacy significantly correlated with functionality uMARS subdomain ratings (Rho = 0.18; P < 0.042). Preferred time of app usage significantly correlated with engagement (Rho = 0.20; P < 0.024), functionality (Rho = 0.19; P < 0.029), total uMARS score (Rho = 0.21; P < 0.017) and subjective quality score (Rho = 0.21; P < 0.017). The vast majority of rheumatic patients would consider recommending Rheuma Auszeit to other patients (117/126; 92.9%).
Conclusion: Rheuma Auszeit was well-accepted by German patients suffering from rheumatoid arthritis, psoriatic arthritis and ankylosing spondyloarthritis. Lacking customization could lead to low app compliance and should be improved. Lower functionality scores among older female rheumatic patients highlight the need for patient education. The study underlines the potential and feasibility of therapeutic complementary digital solutions in rheumatology.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: found
Mobile App Rating Scale: A New Tool for Assessing the Quality of Health Mobile Apps
- Record: found
- Abstract: found
- Article: not found
eHEALS: The eHealth Literacy Scale
- Record: found
- Abstract: found
- Article: found