- Record: found
- Abstract: found
- Article: found
A Phase 3, Randomized, Double-Blind, Comparator-Controlled Study to Evaluate Safety, Tolerability, and Immunogenicity of V114, a 15-Valent Pneumococcal Conjugate Vaccine, in Allogeneic Hematopoietic Cell Transplant Recipients (PNEU-STEM)

Read this article at
Abstract
Background
Individuals who receive allogeneic hematopoietic cell transplant (allo-HCT) are immunocompromised and at high risk of pneumococcal infections, especially in the months following transplant. This study evaluated the safety and immunogenicity of V114 (VAXNEUVANCE; Merck, Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA), a 15-valent pneumococcal conjugate vaccine (PCV), when given to allo-HCT recipients.
Methods
Participants received 3 doses of V114 or PCV13 (Prevnar 13; Wyeth LLC) in 1-month intervals starting 3–6 months after allo-HCT. Twelve months after HCT, participants received either PNEUMOVAX 23 or a fourth dose of PCV (if they experienced chronic graft vs host disease). Safety was evaluated as the proportion of participants with adverse events (AEs). Immunogenicity was evaluated by measuring serotype-specific immunoglobulin G (IgG) geometric mean concentrations (GMCs) and opsonophagocytic activity (OPA) geometric mean titers (GMTs) for all V114 serotypes in each vaccination group.
Results
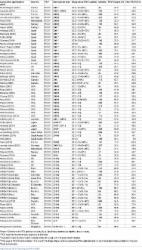
A total of 274 participants were enrolled and vaccinated in the study. The proportions of participants with AEs and serious AEs were generally comparable between intervention groups, and the majority of AEs in both groups were of short duration and mild-to-moderate intensity. For both IgG GMCs and OPA GMTs, V114 was generally comparable to PCV13 for the 13 shared serotypes, and higher for serotypes 22F and 33F at day 90.
Conclusions
V114 was well tolerated in allo-HCT recipients, with a generally comparable safety profile to PCV13. V114 induced comparable immune responses to PCV13 for the 13 shared serotypes, and was higher for V114 serotypes 22F and 33F. Study results support the use of V114 in allo-HCT recipients.
Clinical Trials Registration. clinicaltrials.gov (NCT03565900) and European Union at EudraCT 2018-000066-11.
Abstract
V114, a 15-valent pneumococcal conjugate vaccine, was well tolerated in allogeneic hematopoietic cell transplant recipients with a generally comparable safety profile to PCV13. Immune responses were induced to all 15 serotypes. Study results support V114 use in this at-risk population.
Graphical Abstract

This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/a-phase-3-randomized-double-blind-comparator-controlled-study-to-evaluate-safety-tolerability-and-immunogenicity-of-v114-a-15-valent-pneumococcal-conjugate-vaccine-in-allogeneic-hematopoietic-cell-transplant-recipients-pneu-stem
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
2013 IDSA clinical practice guideline for vaccination of the immunocompromised host.
- Record: found
- Abstract: found
- Article: found