- Record: found
- Abstract: found
- Article: not found
Helicobacter pylori pathogen regulates p14ARF tumor suppressor and autophagy in gastric epithelial cells
Read this article at
Abstract
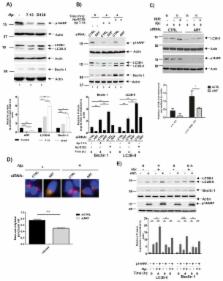
Infection with H. pylori pathogen is one of the strongest risk factors for development of gastric cancer. Although these bacteria infect approximately half of the world’s population, only a small fraction of infected individuals develops gastric malignancies. Interactions between host and bacterial virulence factors are complex and interrelated making it difficult to elucidate specific processes associated with H. pylori-induced tumorigenesis. In this study, we found that H. pylori inhibits p14ARF tumor suppressor by inducing its degradation. This effect was found to be strain-specific. Downregulation of p14ARF induced by H. pylori leads to inhibition of autophagy in a p53-independent manner in infected cells. We identified TRIP12 protein as E3 ubiquitin ligase that is upregulated by H. pylori, inducing ubiquitination and subsequent degradation of p14ARF protein. Using isogenic H. pylori mutants, we found that induction of TRIP12 is mediated by bacterial virulence factor CagA. Increased expression of TRIP12 protein was found in infected gastric epithelial cells in vitro and human gastric mucosa of H. pylori-infected individuals.
In conclusion, our data demonstrate a new mechanism of ARF inhibition that may affect host-bacteria interactions and facilitate tumorigenic transformation in the stomach.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: found
Stromal gene expression predicts clinical outcome in breast cancer.
- Record: found
- Abstract: found
- Article: not found
Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse.
- Record: found
- Abstract: found
- Article: not found
