- Record: found
- Abstract: found
- Article: found
Liquid Chromatography–Mass Spectrometry Analysis of Frataxin Proteoforms in Whole Blood as Biomarkers of the Genetic Disease Friedreich’s Ataxia

Read this article at
Abstract
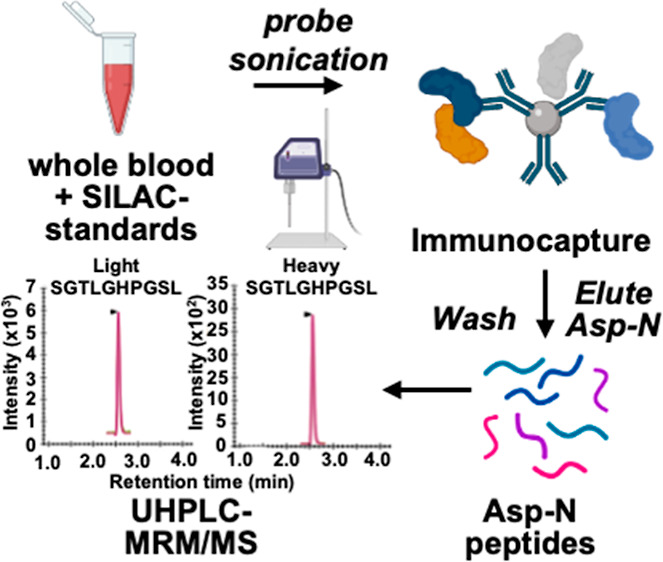
Friedreich’s ataxia (FRDA) is caused primarily by expanded GAA repeats in intron 1 of both alleles of the FXN gene, which causes transcriptional silencing and reduced expression of frataxin mRNA and protein. FRDA is characterized by slowly progressive ataxia and cardiomyopathy. Symptoms generally appear during adolescence, and patients slowly progress to wheelchair dependency usually in the late teens or early twenties with death on average in the 4th decade. There are two known mature proteoforms of frataxin. Mitochondrial frataxin (frataxin-M) is a 130-amino acid protein with a molecular weight of 14,268 Da, and there is an alternatively spliced N-terminally acetylated 135-amino acid form (frataxin-E) with a molecular weight of 14,953 Da found in erythrocytes. There is reduced expression of frataxin in the heart and brain, but frataxin is not secreted into the systemic circulation, so it cannot be analyzed in serum or plasma. Blood is a readily accessible biofluid that contains numerous different cell types that express frataxin. We have found that pig blood can serve as an excellent surrogate matrix to validate an assay for frataxin proteoforms because pig frataxin is lost during the immunoprecipitation step used to isolate human frataxin. Frataxin-M is expressed in blood cells that contain mitochondria, whereas extra-mitochondrial frataxin-E is found in erythrocytes. This means that the analysis of frataxin in whole blood provides information on the concentration of both proteoforms without having to isolate the individual cell types. In the current study, we observed that the distributions of frataxin levels for a sample of 25 healthy controls and 50 FRDA patients were completely separated from each other, suggesting 100% specificity and 100% sensitivity for distinguishing healthy controls from FRDA cases, a very unusual finding for a biomarker assay. Additionally, frataxin levels were significantly correlated with the GAA repeat length and age of onset with higher correlations for extra-mitochondrial frataxin-E than those for mitochondrial frataxin-M. These findings auger well for using frataxin levels measured by the validated stable isotope dilution ultrahigh-performance liquid chromatography–multiple reaction monitoring/mass spectrometry assay to monitor therapeutic interventions and the natural history of FRDA. Our study also illustrates the utility of using whole blood for protein disease biomarker discovery and validation.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Skyline: an open source document editor for creating and analyzing targeted proteomics experiments.

- Record: found
- Abstract: found
- Article: found
Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019
- Record: found
- Abstract: found
- Article: not found