- Record: found
- Abstract: found
- Article: found
Cordycepin‐induced unfolded protein response‐dependent cell death, and AKT/MAPK‐mediated drug resistance in mouse testicular tumor cells
Read this article at
Abstract
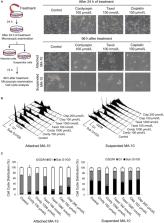
Testicular cancer is the most commonly diagnosed cancer in men at 15‐44 years of age, and radical orchidectomy combined with chemotherapy is currently considered as the standard treatment. However, drugs resistance and side effects that impact the quality of life for patients with testicular cancer have not been markedly improved in recent decades. In this study, we characterized the pharmacological exacerbation of the unfolded protein response (UPR), which is an effective approach to kill testicular cancer cells, by carrying out a clustering analysis of mRNA expression profiles and the immunobloting examination of cordycepin‐treated MA‐10 cells. The UPR is executed in response to endoplasmic reticulum stress to complement by an apoptotic response if the defect cannot be resolved. Results showed that cordycepin significantly modulated FoxO/P15/P27, PERK‐eIF2α (apoptotic), and the IRE1‐XBP1 (adaptive) UPR pathways. Interestingly, a fraction of MA‐10 cells survived after cordycepin treatment, the AKT, LC3 I/II, and MAPK signaling pathways were highly induced in attached cells as compared to the suspended cells, illustrating the drug resistance to cordycepin via activating AKT and MAPK pathways in MA‐10 cells. In summary, PERK‐eIF2α signaling pathway is required for pro‐apoptotic UPR in MA‐10 cell death following cordycepin treatment, suggesting a potential therapeutic application in treating testicular cancer. However, activation of AKT and MAPK pathways could possibly result in drug resistance to cordycepin in MA‐10 cells.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
FOXOs, cancer and regulation of apoptosis.
- Record: found
- Abstract: found
- Article: not found
FOXO transcription factors in cancer development and therapy.
- Record: found
- Abstract: found
- Article: not found