- Record: found
- Abstract: found
- Article: found
Efficacy of alginate-based reflux suppressant and magnesium-aluminium antacid gel for treatment of heartburn in pregnancy: a randomized double-blind controlled trial
Read this article at
Abstract
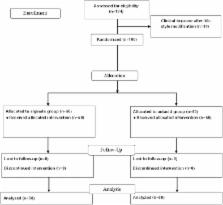
The aim of this study was to compare the therapeutic efficacy of alginate-based reflux suppressant and magnesium-aluminium antacid gel for treatment of heartburn in pregnancy. A double-blinded, randomized, controlled trial was conducted. One hundred pregnant women at less than 36 weeks gestation with heartburn at least twice per week were randomized to either alginate-based reflux suppressant or to magnesium-aluminium antacid gel. Details of heartburn were recorded before beginning the treatment and the second week of study. Primary outcome measure was the improvement of heartburn frequency after treatment and secondary outcome were the improvement of heartburn intensity, quality of life, maternal satisfaction, maternal side effects, pregnancy and neonatal outcomes. There was no difference between treatment and control groups in improvement of heartburn frequency (80% vs 88%, p = 0.275), 50% reduction of frequency of heartburn (56% vs 52%, p = 0.688), improvement of heartburn intensity (92% vs 92%, p = 1.000) and 50% reduction of heartburn intensity (68% vs 80% cases, p = 0.075). There were also no significant differences in quality of life, maternal satisfaction, maternal side effects, pregnancy and neonatal outcomes. Alginate-based reflux suppressant was not different from magnesium-aluminium antacid gel in the treatment of heartburn in pregnancy.
Related collections
Most cited references12
- Record: found
- Abstract: found
- Article: not found
Review article: alginate-raft formulations in the treatment of heartburn and acid reflux.
- Record: found
- Abstract: found
- Article: not found
Review article: the management of heartburn in pregnancy.
- Record: found
- Abstract: found
- Article: not found