- Record: found
- Abstract: found
- Article: found
Radiation nanomedicines for cancer treatment: a scientific journey and view of the landscape
Read this article at
Abstract
Background
Radiation nanomedicines are nanoparticles labeled with radionuclides that emit α- or β-particles or Auger electrons for cancer treatment. We describe here our 15 years scientific journey studying locally-administered radiation nanomedicines for cancer treatment. We further present a view of the radiation nanomedicine landscape by reviewing research reported by other groups.
Main body
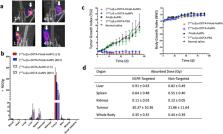
Gold nanoparticles were studied initially for radiosensitization of breast cancer to X-radiation therapy. These nanoparticles were labeled with 111In to assess their biodistribution after intratumoural vs. intravenous injection. Intravenous injection was limited by high liver and spleen uptake and low tumour uptake, while intratumoural injection provided high tumour uptake but low normal tissue uptake. Further, [ 111In]In-labeled gold nanoparticles modified with trastuzumab and injected iintratumourally exhibited strong tumour growth inhibition in mice with subcutaneous HER2-positive human breast cancer xenografts. In subsequent studies, strong tumour growth inhibition in mice was achieved without normal tissue toxicity in mice with human breast cancer xenografts injected intratumourally with gold nanoparticles labeled with β-particle emitting 177Lu and modified with panitumumab or trastuzumab to specifically bind EGFR or HER2, respectively. A nanoparticle depot (nanodepot) was designed to incorporate and deliver radiolabeled gold nanoparticles to tumours using brachytherapy needle insertion techniques. Treatment of mice with s.c. 4T1 murine mammary carcinoma tumours with a nanodepot incorporating [ 90Y]Y-labeled gold nanoparticles inserted into one tumour arrested tumour growth and caused an abscopal growth-inhibitory effect on a distant second tumour. Convection-enhanced delivery of [ 177Lu]Lu-AuNPs to orthotopic human glioblastoma multiforme (GBM) tumours in mice arrested tumour growth without normal tissue toxicity. Other groups have explored radiation nanomedicines for cancer treatment in preclinical animal tumour xenograft models using gold nanoparticles, liposomes, block copolymer micelles, dendrimers, carbon nanotubes, cellulose nanocrystals or iron oxide nanoparticles. These nanoparticles were labeled with radionuclides emitting Auger electrons ( 111In, 99mTc, 125I, 103Pd, 193mPt, 195mPt), β-particles ( 177Lu, 186Re, 188Re, 90Y, 198Au, 131I) or α-particles ( 225Ac, 213Bi, 212Pb, 211At, 223Ra). These studies employed intravenous or intratumoural injection or convection enhanced delivery. Local administration of these radiation nanomedicines was most effective and minimized normal tissue toxicity.
Related collections
Most cited references140
- Record: found
- Abstract: found
- Article: not found
Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma
- Record: found
- Abstract: found
- Article: not found