- Record: found
- Abstract: found
- Article: found
Effects of hTERT immortalization on osteogenic and adipogenic differentiation of dental pulp stem cells
Read this article at
Abstract
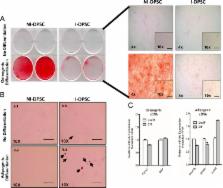
These data relate to the differentiation of human dental pulp stem cells (DPSC) and DPSC immortalized by constitutively expressing human telomerase reverse transcriptase (hTERT) through both osteogenic and adipogenic lineages (i.e. to make bone producing and fat producing cells from these dental pulp stem cells). The data augment another study to characterize immortalized DPSC for the study of neurogenetic “Characterization of neurons from immortalized dental pulp stem cells for the study of neurogenetic disorders” [1]. Two copies of one typical control cell line (technical replicates) were used in this study. The data represent the differentiation of primary DPSC into osteoblast cells approximately 60% more effectively than hTERT immortalized DPSC. Conversely, both primary and immortalized DPSC are poorly differentiated into adipocytes. The mRNA expression levels for both early and late adipogenic and osteogenic gene markers are shown.
Related collections
Most cited references3
- Record: found
- Abstract: found
- Article: not found
Characterization of neurons from immortalized dental pulp stem cells for the study of neurogenetic disorders.
- Record: found
- Abstract: found
- Article: not found
ERα regulates lipid metabolism in bone through ATGL and perilipin.
- Record: found
- Abstract: not found
- Article: not found