- Record: found
- Abstract: found
- Article: found
Alteration of fatty acid oxidation by increased CPT1A on replicative senescence of placenta-derived mesenchymal stem cells
Read this article at
Abstract
Background
Human placenta-derived mesenchymal stem cells (PD-MSCs) are powerful sources for cell therapy in regenerative medicine. However, a limited lifespan by senescence through mechanisms that are well unknown is the greatest obstacle. In the present study, we first demonstrated the characterization of replicative senescent PD-MSCs and their possible mitochondrial functional alterations.
Methods
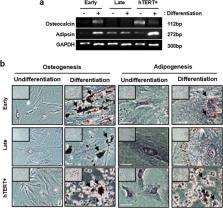
Human PD-MSCs were cultured to senescent cells for a long period of time. The cells of before passage number 8 were early cells and after passage number 14 were late cells. Also, immortalized cells of PD-MSCs (overexpressed hTERT gene into PD-MSCs) after passage number 14 were positive control of non-senescent cells. The characterization and mitochondria analysis of PD-MSCs were explored with long-term cultivation.
Results
Long-term cultivation of PD-MSCs exhibited increases of senescent markers such as SA-β-gal and p21 including apoptotic factor, and decreases of proliferation, differentiation potential, and survival factor. Mitochondrial dysfunction was also observed in membrane potential and metabolic flexibility with enlarged mitochondrial mass. Interestingly, we founded that fatty acid oxidation (FAO) is an important metabolism in PD-MSCs, and carnitine palmitoyltransferase1A (CPT1A) overexpressed in senescent PD-MSCs. The inhibition of CPT1A induced a change of energy metabolism and reversed senescence of PD-MSCs.
Related collections
Most cited references30

- Record: found
- Abstract: found
- Article: found
Mitochondrial effectors of cellular senescence: beyond the free radical theory of aging
- Record: found
- Abstract: found
- Article: not found
Mitochondrial dysfunction contributes to oncogene-induced senescence.
- Record: found
- Abstract: found
- Article: not found