- Record: found
- Abstract: found
- Article: found
Circulating Tumour Cells (CTC), Head and Neck Cancer and Radiotherapy; Future Perspectives
Read this article at
Abstract
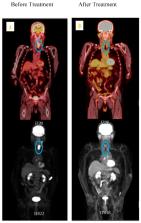
Head and neck cancer is the seventh most common cancer in Australia and globally. Despite the current improved treatment modalities, there is still up to 50–60% local regional recurrence and or distant metastasis. High-resolution medical imaging technologies such as PET/CT and MRI do not currently detect the early spread of tumour cells, thus limiting the potential for effective minimal residual detection and early diagnosis. Circulating tumour cells (CTCs) are a rare subset of cells that escape from the primary tumour and enter into the bloodstream to form metastatic deposits or even re-establish themselves in the primary site of the cancer. These cells are more aggressive and accumulate gene alterations by somatic mutations that are the same or even greater than the primary tumour because of additional features acquired in the circulation. The potential application of CTC in clinical use is to acquire a liquid biopsy, by taking a reliable minimally invasive venous blood sample, for cell genotyping during radiotherapy treatment to monitor the decline in CTC detectability, and mutational changes in response to radiation resistance and radiation sensitivity. Currently, very little has been published on radiation therapy, CTC, and circulating cancer stem cells (CCSCs). The prognostic value of CTC in cancer management and personalised medicine for head and neck cancer radiotherapy patients requires a deeper understanding at the cellular level, along with other advanced technologies. With this goal, this review summarises the current research of head and neck cancer CTC, CCSC and the molecular targets for personalised radiotherapy response.
Related collections
Most cited references158
- Record: found
- Abstract: found
- Article: not found
Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases.
- Record: found
- Abstract: found
- Article: not found
Organoid cultures derived from patients with advanced prostate cancer.
- Record: found
- Abstract: not found
- Article: not found