- Record: found
- Abstract: found
- Article: found
Mapping cannabis potency in medical and recreational programs in the United States
Read this article at
Abstract
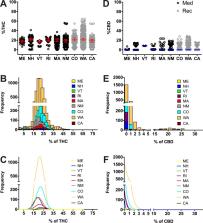
Cannabis related online searches are associated with positive attitudes toward medical cannabis, particularly when information is obtained from dispensaries. Since pain is the main reason for medicinal cannabis use, information from dispensary websites has the potential to shape the attitude of pain patients towards cannabis. This is relevant because cannabis has demonstrated efficacy in neuropathic pain with low tetrahydrocannabinol (THC) concentrations (< 5–10%), in contrast to potent cannabis (>15% THC), which is highly rewarded in the recreational realm. The role of CBD in pain is not clear, however it has gained popularity. Thus, we hypothesize that the potency of medical cannabis that is advertised online is similar to the cannabis advertised for recreational purposes, which would potentially create a misconception towards medical cannabis. The current lack of knowledge surrounding advertised potencies in the legal cannabis market limits the ability to generate clear policies regarding online advertising to protect patients that are willing to use cannabis for their condition. Thus, we evaluated the advertised THC and CBD content of cannabis products offered online in dispensaries in the United States to determine products’ suitability to medicinal use and compare the strength of products offered in legal medical and recreational programs. We recorded THC and CBD concentrations for all herb cannabis products provided by dispensary websites and compared them between or within states. Four Western states (CA, CO, NM, WA) and five Northeastern states (ME, MA, NH, RI, VT) were included. A total of 8,505 cannabis products across 653 dispensaries were sampled. Despite the clear differences between medicinal and recreational uses of cannabis, the average THC concentration advertised online in medicinal programs was similar (19.2% ±6.2) to recreational programs (21.5% ±6.0) when compared between states with different programs, or between medicinal and recreational programs within the same states (CO or WA). Lower CBD concentrations accompanied higher THC products. The majority of products, regardless of medicinal or recreational programs, were advertised to have >15% THC (70.3% - 91.4% of products). These stated concentrations seem unsuitable for medicinal purposes, particularly for patients with chronic neuropathic pain. Therefore, this information could induce the misconception that high potency cannabis is safe to treat pain. This data is consistent with reports in which THC and CBD in products from legal dispensaries or in nationwide products from the illegal market were actually measured, which indicates that patients consuming these products may be at risk of acute intoxication or long-term side effects. Our study offers grounds to develop policies that help prevent misconceptions toward cannabis and reduce risks in pain patients.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial.
- Record: found
- Abstract: found
- Article: not found
Smoked cannabis for chronic neuropathic pain: a randomized controlled trial.
- Record: found
- Abstract: found
- Article: not found