- Record: found
- Abstract: found
- Article: found
Manipulations of MeCP2 in glutamatergic neurons highlight their contributions to Rett and other neurological disorders
Read this article at
Abstract
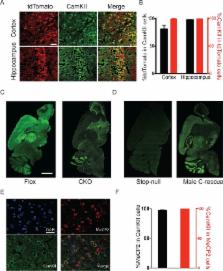
Many postnatal onset neurological disorders such as autism spectrum disorders (ASDs) and intellectual disability are thought to arise largely from disruption of excitatory/inhibitory homeostasis. Although mouse models of Rett syndrome (RTT), a postnatal neurological disorder caused by loss-of-function mutations in MECP2, display impaired excitatory neurotransmission, the RTT phenotype can be largely reproduced in mice simply by removing MeCP2 from inhibitory GABAergic neurons. To determine what role excitatory signaling impairment might play in RTT pathogenesis, we generated conditional mouse models with Mecp2 either removed from or expressed solely in glutamatergic neurons. MeCP2 deficiency in glutamatergic neurons leads to early lethality, obesity, tremor, altered anxiety-like behaviors, and impaired acoustic startle response, which is distinct from the phenotype of mice lacking MeCP2 only in inhibitory neurons. These findings reveal a role for excitatory signaling impairment in specific neurobehavioral abnormalities shared by RTT and other postnatal neurological disorders.
eLife digest
Rett syndrome is a childhood brain disorder that mainly affects girls and causes symptoms including anxiety, tremors, uncoordinated movements and breathing difficulties. Rett syndrome is caused by mutations in a gene called MECP2, which is found on the X chromosome. Males with MECP2 mutations are rare but have more severe symptoms and die young. Many researchers who study Rett syndrome use mice as a model of the disorder. In particular, male mice with the mouse equivalent of the human MECP2 gene switched off in every cell in the body (also known as Mecp2-null mice) show many of the features of Rett syndrome and die at a young age.
The MECP2 gene is important for healthy brain activity. The brain contains two major types of neurons: excitatory neurons, which encourage other neurons to be active; and inhibitory neurons, which stop or dampen the activity of other neurons. In 2010, researchers reported that mice lacking Mecp2 in only their inhibitory neurons develop most of the same problems as those mice with no Mecp2 at all.
Now, Meng et al. – who include two researchers involved in the 2010 study – have asked how deleting or activating Mecp2 only in excitatory neurons of mice affects Rett-syndrome-like symptoms. The experiments showed that male mice without Mecp2 in their excitatory neurons develop tremors, anxiety-like behaviors, abnormal seizure-like brain activity and severe obesity; these mice also die earlier than normal mice. Female mice lacking Mecp2 in half of their excitatory neurons (because the gene is on the X chromosome) were less affected than the males, and had normal life spans. These symptoms are different from those seen in mice missing Mecp2 only in inhibitory neurons.
Meng et al. also found that if Mecp2 was switched on only in excitatory neurons of female mice (which are a model of human Rett syndrome patients) the mice were almost completely normal. In male mice (which show more severe symptoms), activating Mecp2 in only the excitatory neurons reduced the anxiety and tremors. These findings suggest that impaired excitatory neurons may be responsible for specific symptoms such as anxiety and tremors amongst other Rett-syndrome-like features.
The next challenge is to explore how the loss of Mecp2 changes the activity of excitatory neurons in different brain regions. Further studies could also investigate if drugs that improve the activity of excitatory neurons can be used to treat Rett syndrome patients. Finally, in a related study, Ure et al. asked if activating Mecp2 in inhibitory neurons in otherwise Mecp2-null mice was enough to prevent some of their Rett syndrome-like symptoms.
Related collections
Most cited references55
- Record: found
- Abstract: found
- Article: not found
Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2.
- Record: found
- Abstract: found
- Article: not found
A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells.
- Record: found
- Abstract: found
- Article: not found