- Record: found
- Abstract: found
- Article: found
The Clinical Effect of Metformin on the Survival of Lung Cancer Patients with Diabetes: A Comprehensive Systematic Review and Meta-analysis of Retrospective Studies
Read this article at
Abstract
Preclinical investigations have revealed an anti-cancer effect of metformin. Several studies of metformin treatment have demonstrated the improved clinical outcomes of lung cancer patients with diabetes; however, the results have been inconsistent among studies. Our systematic review and meta-analysis aimed to summarize the up-to-date effects of metformin on diabetic lung cancer patients.
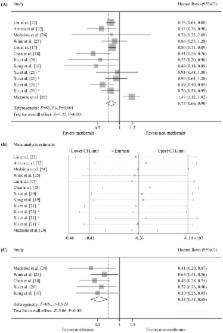
A systematic search was performed for studies published. Then, these studies were evaluated for inclusion, and relevant data was extracted. The summary risk estimates for the associations of metformin treatment with overall survival (OS) and progression-free survival (PFS) were analyzed using random/fixed-effects models. Analyses stratified by histological type were also conducted. Based on the 10 studies included in our analysis, metformin treatment was found to significantly improve survival, corresponding to reductions of 23% and 47% in OS [hazard ratio (HR)=0.77, 95% confidence interval (95%CI)=0.66-0.9, p=0.001] and PFS (HR=0.53, 95%CI=0.41-0.68, p<0.001), respectively. In addition, significant improvements in the OS for non-small cell lung cancer (NSCLC) (HR=0.77, 95%CI=0.71-0.84, p=0.002) and small cell lung cancer (SCLC) (HR=0.52, 95%CI=0.29-0.91, p=0.022) were observed in association with metformin treatment in analysis stratified by histological type. This stratified analysis also revealed a significant improvement in PFS for both NSCLC (HR=0.53, 95%CI=0.39-0.71, p<0.001) and SCLC (HR=0.54, 95%CI=0.34-0.84, p=0.007). We found that metformin treatment significantly improved the OS and PFS of diabetic lung cancer patients, and our findings suggest that metformin might be an effective treatment option for diabetic patients with lung cancer.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: found
Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells.
- Record: found
- Abstract: found
- Article: not found
Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease.
- Record: found
- Abstract: found
- Article: not found