- Record: found
- Abstract: found
- Article: found
Cellular Senescence Affects Cardiac Regeneration and Repair in Ischemic Heart Disease
Read this article at
Abstract
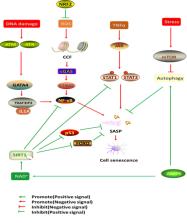
Ischemic heart disease (IHD) is defined as a syndrome of ischemic cardiomyopathy. Myogenesis and angiogenesis in the ischemic myocardium are important for cardiomyocyte (CM) survival, improving cardiac function and decreasing the progression of heart failure after IHD. Cellular senescence is a state of permanent irreversible cell cycle arrest caused by stress that results in a decline in cellular functions, such as proliferation, migration, homing, and differentiation. In addition, senescent cells produce the senescence-associated secretory phenotype (SASP), which affects the tissue microenvironment and surrounding cells by secreting proinflammatory cytokines, chemokines, growth factors, and extracellular matrix degradation proteins. The accumulation of cardiovascular-related senescent cells, including vascular endothelial cells (VECs), vascular smooth muscle cells (VSMCs), CMs and progenitor cells, is an important risk factor of cardiovascular diseases, such as vascular aging, atherosclerotic plaque formation, myocardial infarction (MI) and ventricular remodeling. This review summarizes the processes of angiogenesis, myogenesis and cellular senescence after IHD. In addition, this review focuses on the relationship between cellular senescence and cardiovascular disease and the mechanism of cellular senescence. Finally, we discuss a potential therapeutic strategy for MI targeting senescent cells.
Related collections
Most cited references174

- Record: found
- Abstract: found
- Article: found
The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs
- Record: found
- Abstract: not found
- Article: not found
The serial cultivation of human diploid cell strains.
- Record: found
- Abstract: found
- Article: not found