- Record: found
- Abstract: found
- Article: found
Endo-lysosomal Aβ concentration and pH trigger formation of Aβ oligomers that potently induce Tau missorting
Read this article at
Abstract
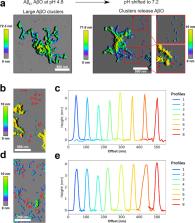
Amyloid-β peptide (Aβ) forms metastable oligomers >50 kDa, termed AβOs, that are more effective than Aβ amyloid fibrils at triggering Alzheimer’s disease-related processes such as synaptic dysfunction and Tau pathology, including Tau mislocalization. In neurons, Aβ accumulates in endo-lysosomal vesicles at low pH. Here, we show that the rate of AβO assembly is accelerated 8,000-fold upon pH reduction from extracellular to endo-lysosomal pH, at the expense of amyloid fibril formation. The pH-induced promotion of AβO formation and the high endo-lysosomal Aβ concentration together enable extensive AβO formation of Aβ42 under physiological conditions. Exploiting the enhanced AβO formation of the dimeric Aβ variant dimAβ we furthermore demonstrate targeting of AβOs to dendritic spines, potent induction of Tau missorting, a key factor in tauopathies, and impaired neuronal activity. The results suggest that the endosomal/lysosomal system is a major site for the assembly of pathomechanistically relevant AβOs.
Abstract
Aβ oligomers (AβO) are thought to represent the main toxic species in Alzheimer’s disease but very high Aβ concentrations are required to study them in vitro and it remains unknown what role these off-pathway oligomers play in vivo. Here, the authors use a dimeric variant of Aβ termed dimAβ, where two Aβ40 units are linked, which facilitates to study AβO formation kinetics and they observe that Aβ off-pathway oligomer formation is strongly accelerated at endo-lysosomal pH, while amyloid fibril formation is delayed. Furthermore, the authors demonstrate that dimAβ is a disease-relevant model construct for pathogenic AβO formation by showing that dimAβ AβOs target dendritic spines and induce AD-like somatodendritic Tau missorting.
Related collections
Most cited references78
- Record: found
- Abstract: found
- Article: not found
Fiji: an open-source platform for biological-image analysis.

- Record: found
- Abstract: found
- Article: found