- Record: found
- Abstract: found
- Article: found
Inhibition of Rho-Kinase Downregulates Th17 Cells and Ameliorates Hepatic Fibrosis by Schistosoma japonicum Infection
Read this article at
Abstract
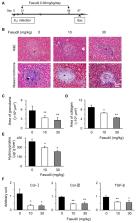
Background: Schistosomiasis is an immunopathogenic disease in which Th17 cells play vital roles. Hepatic granuloma formation and subsequent fibrosis are its main pathologic manifestations and the leading causes of hepatic cirrhosis, and effective therapeutic interventions are lacking. In this study, we explored the effects of fasudil, a selective RhoA–Rho-associated kinase (ROCK) inhibitor, on Th17 cells and the pathogenesis of schistosomiasis. Methods: Mice were infected with Schistosoma japonicum and treated with fasudil. The worm burden, hepatic granuloma formation, and fibrosis were evaluated. The roles of fasudil on Th17, Treg, and hepatic stellate cells were analyzed. Results: Fasudil therapy markedly reduced the granuloma size and collagen deposit in livers from mice infected with S. japonicum. However, fasudil therapy did not affect the worm burden in infected mice. The underlying cellular and molecular mechanisms were investigated. Fasudil suppressed the activation and induced the apoptosis of CD4 + T cells. Fasudil inhibited the differentiation and effector cytokine secretion of Th17 cells, whereas it upregulated Treg cells in vitro. It also restrained the in vivo interleukin (IL)-4 and IL-17 levels in infected mice. Fasudil directly induced the apoptosis of hepatic stellate cells and downregulated the expressions of hepatic fibrogenic genes, such as collagen type I ( Col-I), Col-III, and transforming growth factor-1 ( TGF-β1). These effects may contribute to its anti-pathogenic roles in schistosomiasis. Conclusions: Fasudil inhibits hepatic granuloma formation and fibrosis with downregulation of Th17 cells. Fasudil might serve as a novel therapeutic agent for hepatic fibrosis due to schistosome infections and perhaps other disorders.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Schistosomiasis
- Record: found
- Abstract: found
- Article: not found
Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice.
- Record: found
- Abstract: found
- Article: not found