- Record: found
- Abstract: found
- Article: not found
Practical High-Throughput Experimentation for Chemists
rapid-communication

Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
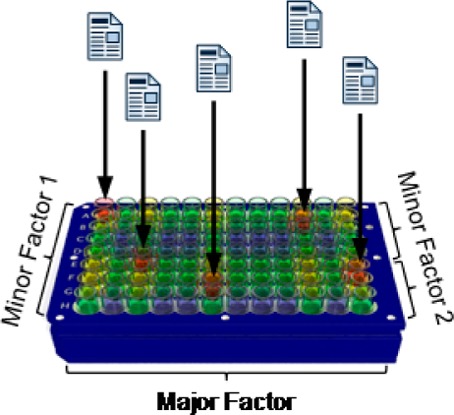
Large arrays of hypothesis-driven, rationally designed experiments are powerful tools for solving complex chemical problems. Conceptual and practical aspects of chemical high-throughput experimentation are discussed. A case study in the application of high-throughput experimentation to a key synthetic step in a drug discovery program and subsequent optimization for the first large scale synthesis of a drug candidate is exemplified.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Discovery of an α-amino C-H arylation reaction using the strategy of accelerated serendipity.
- Record: found
- Abstract: found
- Article: not found
Organic chemistry. Nanomole-scale high-throughput chemistry for the synthesis of complex molecules.
- Record: found
- Abstract: found
- Article: not found
A robustness screen for the rapid assessment of chemical reactions.
Karl Collins, Frank Glorius (2013)
