- Record: found
- Abstract: found
- Article: found
Profiling the Plasmodium falciparum Erythrocyte Membrane Protein 1–Specific Immununoglobulin G Response Among Ghanaian Children With Hemoglobin S and C
Read this article at
Abstract
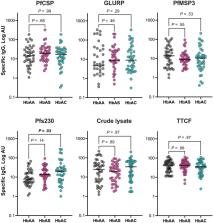
Members of the Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) family are important targets for protective immunity. Abnormal display of PfEMP1 on the surfaces of infected erythrocytes (IEs) and reduced cytoadhesion have been demonstrated in hemoglobin (Hb) AS and HbAC, inherited blood disorders associated with protection against severe P. falciparum malaria. We found that Ghanaian children with HbAS had lower levels of immunoglobulin G against several PfEMP1 variants and that this reactivity increased more slowly with age than in their HbAA counterparts. Moreover, children with HbAS have lower total parasite biomass than those with HbAA at comparable peripheral parasitemias, suggesting impaired cytoadhesion of HbAS IEs in vivo and likely explaining the slower acquisition of PfEMP1-specific immunoglobulin G in this group. In contrast, the function of acquired antibodies was comparable among Hb groups and appears to be intact and sufficient to control parasitemia via opsonization and phagocytosis of IEs.
Abstract
Heterozygous carriers of sickle hemoglobin (HbAS) had reduced total and sequestered parasite biomass and lower immunoglobulin G response to Plasmodium falciparum erythrocyte membrane protein 1 variants associated with severe malaria. Moreover, reactivity increased more slowly with age in those individuals.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity.
- Record: found
- Abstract: found
- Article: not found
Classification of adhesive domains in the Plasmodium falciparum erythrocyte membrane protein 1 family.
- Record: found
- Abstract: found
- Article: not found