- Record: found
- Abstract: found
- Article: found
A Sweet Talk: The Molecular Systems of Perineuronal Nets in Controlling Neuronal Communication
Read this article at
Abstract
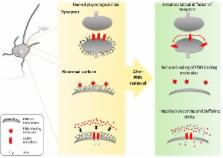
Perineuronal nets (PNNs) are mesh-like structures, composed of a hierarchical assembly of extracellular matrix molecules in the central nervous system (CNS), ensheathing neurons and regulating plasticity. The mechanism of interactions between PNNs and neurons remain uncharacterized. In this review, we pose the question: how do PNNs regulate communication to and from neurons? We provide an overview of the current knowledge on PNNs with a focus on the cellular interactions. PNNs ensheath a subset of the neuronal population with distinct molecular aspects in different areas of the CNS. PNNs control neuronal communication through molecular interactions involving specific components of the PNNs. This review proposes that the PNNs are an integral part of neurons, crucial for the regulation of plasticity in the CNS.
Related collections
Most cited references68
- Record: found
- Abstract: found
- Article: not found
Perineuronal nets protect fear memories from erasure.
- Record: found
- Abstract: found
- Article: not found