- Record: found
- Abstract: found
- Article: found
Pom1 regulates the assembly of Cdr2–Mid1 cortical nodes for robust spatial control of cytokinesis
Read this article at
Abstract
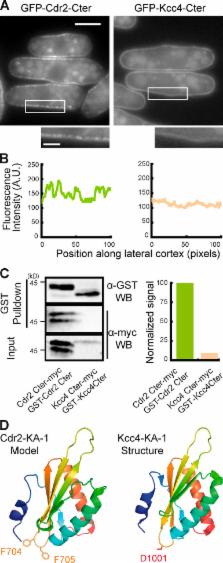
Pom1 regulation of Cdr2 membrane association and interaction with Mid1 prevents Cdr2 assembly into stable nodes in the cell tip region, which ensures proper positioning of cytokinetic ring precursors and accurate division plane positioning in fission yeast.
Abstract
Proper division plane positioning is essential to achieve faithful DNA segregation and to control daughter cell size, positioning, or fate within tissues. In Schizosaccharomyces pombe, division plane positioning is controlled positively by export of the division plane positioning factor Mid1/anillin from the nucleus and negatively by the Pom1/DYRK (dual-specificity tyrosine-regulated kinase) gradients emanating from cell tips. Pom1 restricts to the cell middle cortical cytokinetic ring precursor nodes organized by the SAD-like kinase Cdr2 and Mid1/anillin through an unknown mechanism. In this study, we show that Pom1 modulates Cdr2 association with membranes by phosphorylation of a basic region cooperating with the lipid-binding KA-1 domain. Pom1 also inhibits Cdr2 interaction with Mid1, reducing its clustering ability, possibly by down-regulation of Cdr2 kinase activity. We propose that the dual regulation exerted by Pom1 on Cdr2 prevents Cdr2 assembly into stable nodes in the cell tip region where Pom1 concentration is high, which ensures proper positioning of cytokinetic ring precursors at the cell geometrical center and robust and accurate division plane positioning.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Membrane recognition by phospholipid-binding domains.
- Record: found
- Abstract: found
- Article: not found
Cytokinesis in animal cells.
- Record: found
- Abstract: found
- Article: not found