- Record: found
- Abstract: found
- Article: found
Inflammation and diabetic retinal microvascular complications
review-article
Wenbo Zhang
1
,
2
,
3 ,
Hua Liu
1
,
2 ,
Mohamed Al-Shabrawey
2
,
4 ,
Robert W. Caldwell
3 ,
Ruth B. Caldwell
1
,
2
,
5
,
6
,
7
Apr-Jun 2011
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
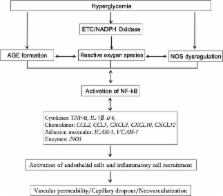
Diabetic retinopathy (DR) is one of the most common complications of diabetes and is a leading cause of blindness in people of the working age in Western countries. A major pathology of DR is microvascular complications such as non-perfused vessels, microaneurysms, dot/blot hemorrhages, cotton-wool spots, venous beading, vascular loops, vascular leakage and neovascularization. Multiple mechanisms are involved in these alternations. This review will focus on the role of inflammation in diabetic retinal microvascular complications and discuss the potential therapies by targeting inflammation.
Related collections
Most cited references82
- Record: found
- Abstract: found
- Article: not found
A central role for inflammation in the pathogenesis of diabetic retinopathy.
Antonia Joussen, Vassiliki Poulaki, Minh Ly Le … (2004)
- Record: found
- Abstract: found
- Article: not found
Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction.
- Record: found
- Abstract: found
- Article: not found
Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis.
Kip Connor, John SanGiovanni, Chatarina Löfqvist … (2007)