- Record: found
- Abstract: found
- Article: found
Accurate and efficient amino acid analysis for protein quantification using hydrophilic interaction chromatography coupled tandem mass spectrometry
Read this article at
Abstract
Background
Methods used to quantify protein from biological samples are often inaccurate with significant variability that requires care to minimize. The errors result from losses during protein preparation and purification and false detection of interfering compounds or elements. Amino acid analysis (AAA) involves a series of chromatographic techniques that can be used to measure protein levels, avoiding some difficulties and providing specific compositional information. However, unstable derivatives, that are toxic and can be costly, incomplete reactions, inadequate chromatographic separations, and the lack of a single hydrolysis method with sufficient recovery of all amino acids hinder precise protein quantitation using AAA.
Results
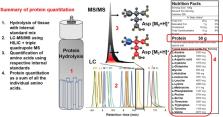
In this study, a hydrophilic interaction chromatography based method was used to separate all proteinogenic amino acids, including isobaric compounds leucine and isoleucine, prior to detection by multiple reaction monitoring with LC–MS/MS. Through inclusion of commercially available isotopically labeled ( 13C, 15N) amino acids as internal standards we adapted an isotopic dilution strategy for amino acid-based quantification of proteins. Three hydrolysis methods were tested with ubiquitin, bovine serum albumin, (BSA), and a soy protein biological reference material (SRM 3234; NIST) resulting in protein estimates that were 86–103%, 82–94%, and 90–99% accurate for the three protein samples respectively. The methane sulfonic acid hydrolysis approach provided the best recovery of labile amino acids including: cysteine, methionine and tryptophan that are challenging to accurately quantify.
Conclusions
Accurate determination of protein quantity and amino acid composition in heterogeneous biological samples is non-trivial. Recent advances in chromatographic phases and LC–MS/MS based methods, along with the availability of isotopic standards can minimize difficulties in analysis and improve protein quantitation. A robust method is described for high-throughput protein quantification and amino acid compositional analysis. Since accurate measurement of protein quality and quantity are a requirement for many biological studies that relate to crop improvement or more generally, our understanding of metabolism in living systems, we envision this method will have broad applicability.
Related collections
Most cited references55
- Record: found
- Abstract: not found
- Article: not found
Automatic Recording Apparatus for Use in Chromatography of Amino Acids
- Record: found
- Abstract: found
- Article: not found
Converting nitrogen into protein--beyond 6.25 and Jones' factors.
- Record: found
- Abstract: found
- Article: not found