- Record: found
- Abstract: found
- Article: found
1,2,3,4,6‐Penta‐O‐galloyl‐β‐d‐glucose modulates perivascular inflammation and prevents vascular dysfunction in angiotensin II‐induced hypertension
Read this article at
Abstract
Background and Purpose
Hypertension is a multifactorial disease, manifested by vascular dysfunction, increased superoxide production, and perivascular inflammation. In this study, we have hypothesized that 1,2,3,4,6‐penta‐ O‐galloyl‐β‐ d‐glucose (PGG) would inhibit vascular inflammation and protect from vascular dysfunction in an experimental model of hypertension.
Experimental Approach
PGG was administered to mice every 2 days at a dose of 10 mg·kg −1 i.p during 14 days of Ang II infusion. It was used at a final concentration of 20 μM for in vitro studies in cultured cells.
Key Results
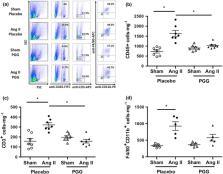
Ang II administration increased leukocyte and T‐cell content in perivascular adipose tissue (pVAT), and administration of PGG significantly decreased total leukocyte and T‐cell infiltration in pVAT. This effect was observed in relation to all T‐cell subsets. PGG also decreased the content of T‐cells bearing CD25, CCR5, and CD44 receptors and the expression of both monocyte chemoattractant protein 1 (CCL2) in aorta and RANTES (CCL5) in pVAT. PGG administration decreased the content of TNF + and IFN‐γ + CD8 T‐cells and IL‐17A + CD4 + and CD3 +CD4 −CD8 − cells. Importantly, these effects of PGG were associated with improved vascular function and decreased ROS production in the aortas of Ang II‐infused animals independently of the BP increase. Mechanistically, PGG (20 μM) directly inhibited CD25 and CCR5 expression in cultured T‐cells. It also decreased the content of IFN‐γ + CD8 + and CD3 +CD4 −CD8 − cells and IL‐17A + CD3 +CD4 −CD8 − cells.
Conclusion and Implication
PGG may constitute an interesting immunomodulating strategy in the regulation of vascular dysfunction and hypertension.
Linked Articles
This article is part of a themed section on Immune Targets in Hypertension. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.12/issuetoc
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction.
- Record: found
- Abstract: found
- Article: not found
Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial.
- Record: found
- Abstract: found
- Article: not found