- Record: found
- Abstract: found
- Article: found
Recent Advances in CRISPR/Cas9 Delivery Strategies
Read this article at
Abstract
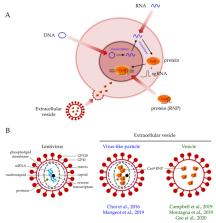
The clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system has revolutionized the field of gene editing. Continuous efforts in developing this technology have enabled efficient in vitro, ex vivo, and in vivo gene editing through a variety of delivery strategies. Viral vectors are commonly used in in vitro, ex vivo, and in vivo delivery systems, but they can cause insertional mutagenesis, have limited cloning capacity, and/or elicit immunologic responses. Physical delivery methods are largely restricted to in vitro and ex vivo systems, whereas chemical delivery methods require extensive optimization to improve their efficiency for in vivo gene editing. Achieving a safe and efficient in vivo delivery system for CRISPR/Cas9 remains the most challenging aspect of gene editing. Recently, extracellular vesicle-based systems were reported in various studies to deliver Cas9 in vitro and in vivo. In comparison with other methods, extracellular vesicles offer a safe, transient, and cost-effective yet efficient platform for delivery, indicating their potential for Cas9 delivery in clinical trials. In this review, we first discuss the pros and cons of different Cas9 delivery strategies. We then specifically review the development of extracellular vesicle-mediated gene editing and highlight the strengths and weaknesses of this technology.
Related collections
Most cited references62
- Record: found
- Abstract: found
- Article: not found
Efficient Delivery of Genome-Editing Proteins In Vitro and In Vivo
- Record: found
- Abstract: found
- Article: not found
Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients.
- Record: found
- Abstract: found
- Article: not found