- Record: found
- Abstract: found
- Article: found
Endothelial microparticles reduce ICAM-1 expression in a microRNA-222-dependent mechanism
Read this article at
Abstract
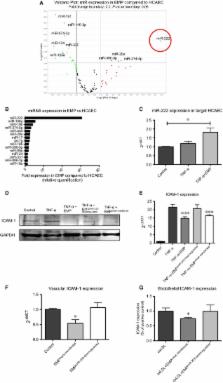
Endothelial microparticles (EMP) are released from activated or apoptotic endothelial cells (ECs) and can be taken up by adjacent ECs, but their effect on vascular inflammation after engulfment is largely unknown. We sought to determine the role of EMP in EC inflammation. In vitro, EMP treatment significantly reduced tumour necrosis factor-α-induced endothelial intercellular adhesion molecule (ICAM)-1 expression on mRNA and protein level, whereas there was no effect on vascular cell adhesion molecule-1 expression. Reduced ICAM-1 expression after EMP treatment resulted in diminished monocyte adhesion in vitro. In vivo, systemic treatment of ApoE−/− mice with EMP significantly reduced murine endothelial ICAM-1 expression. To explore the underlying mechanisms, Taqman microRNA array was performed and microRNA (miR)-222 was identified as the strongest regulated miR between EMP and ECs. Following experiments demonstrated that miR-222 was transported into recipient ECs by EMP and functionally regulated expression of its target protein ICAM-1 in vitro and in vivo. After simulating diabetic conditions, EMP derived from glucose-treated ECs contained significantly lower amounts of miR-222 and showed reduced anti-inflammatory capacity in vitro and in vivo. Finally, circulating miR-222 level was diminished in patients with coronary artery disease (CAD) compared to patients without CAD. EMPs promote anti-inflammatory effects in vitro and in vivo by reducing endothelial ICAM-1 expression via the transfer of functional miR-222 into recipient cells. In pathological hyperglycaemic conditions, EMP-mediated miR-222-dependent anti-inflammatory effects are reduced.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia.
- Record: found
- Abstract: found
- Article: not found
Microparticles, vascular function, and atherothrombosis.
- Record: found
- Abstract: found
- Article: not found