- Record: found
- Abstract: found
- Article: found
Maternal Immune Activation Induces Neuroinflammation and Cortical Synaptic Deficits in the Adolescent Rat Offspring
Read this article at
Abstract
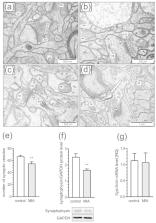
Maternal immune activation (MIA), induced by infection during pregnancy, is an important risk factor for neuro-developmental disorders, such as autism. Abnormal maternal cytokine signaling may affect fetal brain development and contribute to neurobiological and behavioral changes in the offspring. Here, we examined the effect of lipopolysaccharide-induced MIA on neuro-inflammatory changes, as well as synaptic morphology and key synaptic protein level in cerebral cortex of adolescent male rat offspring. Adolescent MIA offspring showed elevated blood cytokine levels, microglial activation, increased pro-inflammatory cytokines expression and increased oxidative stress in the cerebral cortex. Moreover, pathological changes in synaptic ultrastructure of MIA offspring was detected, along with presynaptic protein deficits and down-regulation of postsynaptic scaffolding proteins. Consequently, ability to unveil MIA-induced long-term alterations in synapses structure and protein level may have consequences on postnatal behavioral changes, associated with, and predisposed to, the development of neuropsychiatric disorders.
Related collections
Most cited references81
- Record: found
- Abstract: found
- Article: not found
Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism.
- Record: found
- Abstract: found
- Article: not found
Elevated immune response in the brain of autistic patients.

- Record: found
- Abstract: found
- Article: found