- Record: found
- Abstract: found
- Article: found
Integrating genetic and immune factors to uncover pathogenetic mechanisms of viral-associated pulmonary aspergillosis
Read this article at
ABSTRACT
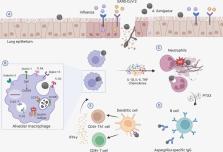
Invasive pulmonary aspergillosis is a severe fungal infection primarily affecting immunocompromised patients. Individuals with severe viral infections have recently been identified as vulnerable to developing invasive fungal infections. Both influenza-associated pulmonary aspergillosis (IAPA) and COVID-19-associated pulmonary aspergillosis (CAPA) are linked to high mortality rates, emphasizing the urgent need for an improved understanding of disease pathogenesis to unveil new molecular targets with diagnostic and therapeutic potential. The recent establishment of animal models replicating the co-infection context has offered crucial insights into the mechanisms that underlie susceptibility to disease. However, the development and progression of human viral-fungal co-infections exhibit a significant degree of interindividual variability, even among patients with similar clinical conditions. This observation implies a significant role for host genetics, but information regarding the genetic basis for viral-fungal co-infections is currently limited. In this review, we discuss how genetic factors known to affect either antiviral or antifungal immunity could potentially reveal pathogenetic mechanisms that predispose to IAPA or CAPA and influence the overall disease course. These insights are anticipated to foster further research in both pre-clinical models and human patients, aiming to elucidate the complex pathophysiology of viral-associated pulmonary aspergillosis and contributing to the identification of new diagnostic and therapeutic targets to improve the management of these co-infections.
Related collections
Most cited references133
- Record: found
- Abstract: found
- Article: not found
Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report
- Record: found
- Abstract: found
- Article: not found
Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19
- Record: found
- Abstract: found
- Article: not found